Plant Pathology Introduction
Importance of the Plant Diseases
Globally, enormous losses of the crops are caused by the plant diseases. The loss can occur from the time of seed sowing in the field to harvesting and storage. Important historical evidences of plant disease epidemics are Irish Famine due to late blight of potato (Ireland, 1845), Bengal famine due to brown spot of rice (India, 1942) and Coffee rust (Sri Lanka, 1967). Such epidemics had left their effect on the economy of the affected countries.Objectives of Plant Pathology
Plant Pathology (Phytopathology) deals with the cause, etiology, resulting losses and control or management of the plant diseases. The objectives of the Plant Pathology are the study on:i. the living entities that cause diseases in plants;
ii. the non-living entities and the environmental conditions that cause disorders in plants;
iii. the mechanisms by which the disease causing agents produce diseases;
iv. the interactions between the disease causing agents and host plant in relation to overall environment; and
v. the method of preventing or management the diseases and reducing the losses/damages caused by diseases.
Scope of Plant Pathology:
Plant pathology comprises with the basic knowledge and technologies of Botany, Plant Anatomy, Plant Physiology, Mycology, Bacteriology, Virology, Nematology, Genetics, Molecular Biology, Genetic Engineering, Biochemistry, Horticulture, Tissue Culture, Soil Science, Forestry, Physics, Chemistry, Meteorology, Statistics and many other branches of applied science.Concept of Plant Disease:
The normal physiological functions of plants are disturbed when they are affected by pathogenic living organisms or by some environmental factors. Initially plants react to the disease causing agents, particularly in the site of infection. Later, the reaction becomes more widespread and histological changes take place. Such changes are expressed as different types of symptoms of the disease which can be visualized macroscopically. As a result of the disease, plant growth in reduced, deformed or even the plant dies.When a plant is suffering, we call it diseased, i.e. it is at ‘dis-ease’. Disease is a condition that occurs in consequence of abnormal changes in the form, physiology, integrity or behaviour of the plant. Disease is a deviation from normal functioning of physiological processes of sufficient duration or intensity to cause disturbance or cessation of vital activities.
A plant is diseased when it is continuously disturbed by some causal agent that results in abnormal physiological process that disrupts the plants normal structure, growth, function or other activities. This interference with one or more plant’s essential physiological or biochemical systems elicites characteristic pathological conditions or symptoms.
Causes of Plant Diseases
Plant diseases are caused by pathogens. Hence a pathogen is always associated with a disease. In other way, disease is a symptom caused by the invasion of a pathogen that is able to survive, perpetuate and spread. Further, the word “pathogen” can be broadly defined as any agent or factor that incites ‘pathos or disease in an organism or host. In strict sense, the causes of plant diseases are grouped under following categories:1. Animate or biotic causes:
Pathogens of living nature are categorized into the following groups.(i) Fungi
(ii) Bacteria
(iii) Phytoplasma
(iv) Rickettsia-like organisms
(v) Algae
(vi) Phanerogams
(vii) Protozoa
(viii) Nematodes
2. Mesobiotic causes :
These disease incitants are neither living or non-living, e.g.
(i) Viruses
(ii) Viroides
3. Inanimate or abiotic causes:
In true sense these factors cause damages (any reduction in the quality or quantity of yield or loss of revenue) to the plants rather than causing disease. The causes are:(i) Deficiencies or excess of nutrients (e.g. ‘Khaira’ disease of rice due to Zn deficiency)
(ii) Light
(iii) Moisture
(iv) Temperature
(v) Air pollutants (e.g. black tip of mango)
(vi) Lack of oxygen (e.g. hollow and black heart of potato)
(vii) Toxicity of pesticides
(viii) Improper cultural practices
(ix) Abnormality in soil conditions (acidity, alkalinity).
Classification of Plant Disease
To facilitate the study of plant diseases they are needed to be grouped in some orderly fashion. Plant diseases can be grouped in various ways based on the symptoms or signs (rust, smut, blight etc.), nature of infection (systemic or localized), habitat of the pathogens, mode of perpetuation and spread (soil-, seed- and air-borne etc.), affected parts of the host (aerial, root disease etc.), types of the plants (cereals, pulses, oilseed, ornamental, vegetable, forest diseases etc.). But the most useful classification has been made based on the type of pathogens that cause plant diseases. Since this type of classification indicates not only the cause of the disease, but also the knowledge and information that suggest the probable development and spread of disease along with their possible control measures. The classification is as follows:1. Infectious plant diseases:
a. Disease caused by parasitic organisms: The organisms included in animate or biotic causes can incite diseases in plants.b. Diseases caused by viruses and viroids.
2. Non-infectious or non-parasitic or physiological diseases:
The factors included in inanimate or abiotic causes can incite such diseases in plants under a set of suitable environmental conditions.Some important terms:
1. Parasite: An organism living upon or in another living organism (the host) and obtaining the food from the invading host.2. Pathogen: An entity, usually a micro-organism that can cause the disease.
3. Biotroph: A plant pathogenic fungus that requires living host cells i.e. an obligate parasite.
4. Hemibiotroph: A plant pathogenic fungus that initially requires living host cells but after killing the host cell grows on the dead and dying cells.
5. Necrotroph: A pathogenic fungus that kills the host and survives on the dying and dead cells.
6. Pathogenicity: The relative capability of a pathogen to cause disease.
7. Pathogenesis: It is a process caused by an infectious agent (pathogen) when it comes in contact with a susceptible host.
8. Virulence: The degree of infectivity of a given pathogen.
9. Infection: The initiation and establishment of a parasite within a host plant.
10. Primary infection: The first infection of a plant by the over wintering or over summering of the pathogen.
11. Inoculum: That portion of pathogen which is transferred to plant and cause disease.
12. Invasion: The penetration and spread of a pathogen in the host.
13. Colonization: The growth of a pathogen, particularly a fungus, in the host after infection is called colonization.
14. Inoculum potential: The growth or threshold of fungus available for colonization at substratum (host).
15. Symptoms: The external and internalreaction or alterations of a plant as a result of disease.
16. Incubation period: The period of timebetween penetration of a pathogen to the host and the first appearance of symptoms on the plant.
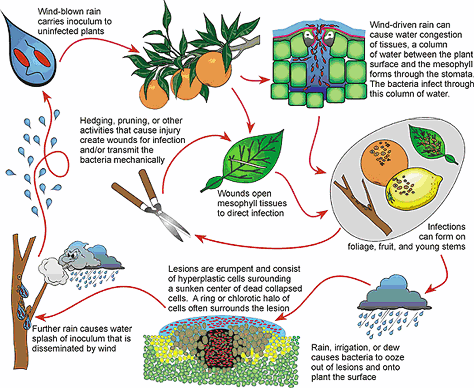
17. Disease cycle: The chain of events involved in disease development.
18. Disease syndrome: The set of varying symptoms characterizing a disease are collectively called a syndrome.
19. Single cycle disease (Monocyclic): This type of disease is referred to those caused by the pathogen (fungi) that can complete only one life cycle in one crop season of the host plant. e.g. downy mildew of rapeseed, club root of crucifers, sclerotinia blight of brinjal etc.
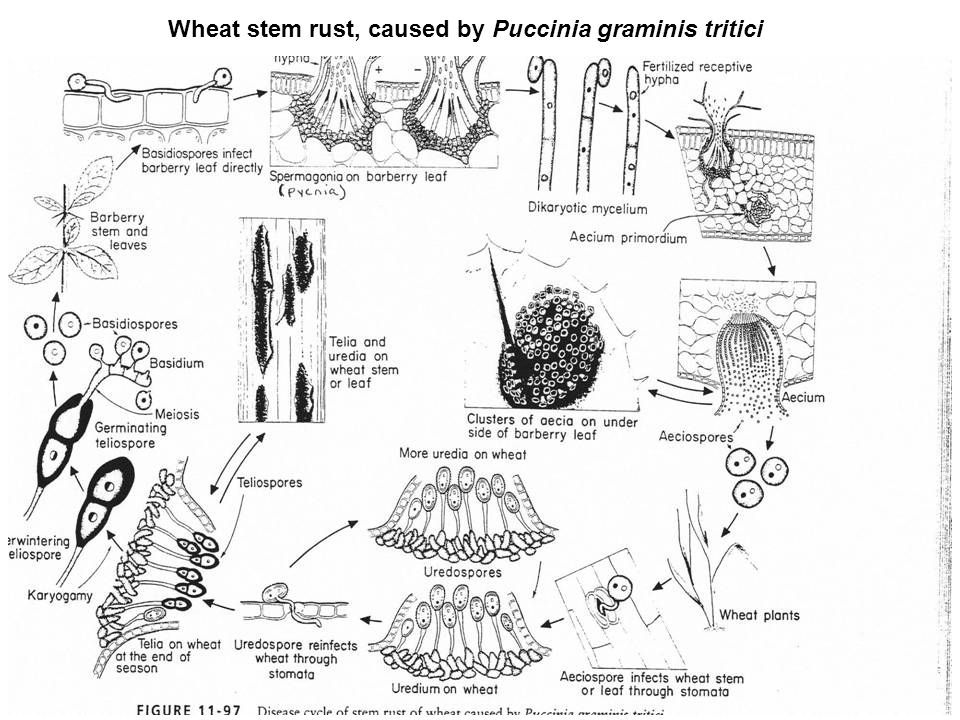
20. Multiple cycle disease (Polycyclic): Some pathogens specially a fungus, can complete a number of life cycles within one crop season of the host plant and the disease caused by such pathogens is called multiple cycle disease e.g. wheat rust, rice blast, late blight of potato etc.
21. Alternate host: Plants not related to the main host of parasitic fungus, where it produces its different stages to complete one cycle (heteroecious).
22. Collateral host: The wild host of same families of a pathogen is called as collateral host.
23. Predisposition: The effect of one or more environmental factors which makes a plant vulnerable to attack by a pathogen.
24. Physiologic race: One or a group of microorganisms similar in morphology but dissimilar in certain cultural, physiological or pathological characters.
25. Biotype: The smallest morphological unit within a species, the members of which are usually genetically identical.
26. Symbiosis: A mutually beneficial association of two or more different kinds of organisms.
27. Mutualism: Symbiosis of two organisms that are mutually helpful or that mutually support one another.
28. Antagonism: The counter action between organisms or groups of organisms.
29. Mutation: An abrupt appearance of a new characteristic in an individual as a result of an accidental change in genes present in chromosomes.
30. Disease: Any deviation in the general health, or physiology or function of plant or plant parts, is recognized as a disease.
31. Cop Damage: It is defined as any reduction in the quality or quantity of yield or loss of revenue resulting from crop injury.
32. Deficiency: Abnormality or disease caused by the lack or subnormal level of availability of one or more essential nutrient elements.
Effect of pathogen on the plants:
During the course of pathogenesis, normal activities of the infected host plant undergo malfunction. Consequently, morphological and physiological changes occur.A. Morphological or structural changes:
Physiological malfunctioning of the host cells causes disturbances in chemical reactionwhich ultimately lead to some structural changes viz., overgrowth, phyllody(abnormal development of floral parts into leafy structures), sterile flowers, hairy roots, witches broom, bunchy top, crown gall, root knot, leaf curling, rolling, puckering etc.B. Physiological changes:
i. Disintegration of the tissues by the enzymesof the pathogen.ii. Effect on the growth of the host plant due to growth regulators produced by the pathogen or by the host under the influence of the pathogen.
iii. Effect on uptake and translocation of water and nutrients.
iv. Abnormality in respiration of the host tissues due to disturbed permeability of cell membrane and enzyme system associated with respiration.
v. Impairing the phenomenon of photosynthesis due to loss of chlorophyll and destruction of leaf tissue.
vi. Effect on the process of translation and transcription.
vii. Overall reproduction system of the host.
Symptoms of Plant Diseases
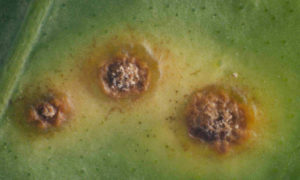
A visible or detectable abnormality expressed on the plant as a result of disease or disorder is called symptom. The totality of symptoms is collectively called as syndrome while the pathogen or its parts or products seen on the affected parts of a host plant is called sign. Different types of disease symptoms are cited below:Necrosis: It indicates the death of cells, tissues and organs resulting from infection by pathogen. Necrotic symptoms include spots, blights, burn, canker, streaks, stripes, damping-off, rot etc.
Wilt: Withering and drooping of a plant starting from some leaves to growing tip occurs suddenly or gradually. Wilting takes place due to blockage in the translocation system caused by the pathogen.
Die-back: Drying of plant organs such as stem or branches which starts from the tip and progresses gradually towards the main stem or trunk is called die-back or wither tip.
Mildew: White, grey or brown coloured superficial growth of the pathogen on the host surface is called mildew.
Rusts: Numerous small pustules growing out through host epidermis which gives rusty (rust formation on iron) appearance of the affected parts.
Smuts: Charcoal-like and black or purplish-black dust like masses developed on the affected plant parts, mostly on floral organs and inflorescens are called smut.
Blotch: A large area of discolouration of a leaf, fruit etc. giving a blotchy appearance.
White blisters: Numerous white coloured blister-like ruptures are surfaced on the host epidermis that forms powdery masses of spores of fungi. They are called white blisters or white rust.
Colour change: It denotes conversion of green pigment of leaves into other colours mostly to yellow colour, in patches or covering the entire leaves.
(i) Etioliation: Yellowing due to lack of light,
(ii) Chlorosis: Yellowing due to infection viruses, bacteria, fungi, low temperature lack of iron etc.
(iii) Albino: Lack of any pigment and turned into white or bleached
(iv) Chromosis: Red, purple or orange pigmentation due to physiological orders etc.
Exudation: Such symptom is commonly found in bacterial diseases when masses of bacterial cells ooze out to the surface of affected plant parts and form some drops or smear, it is called exudation. This exudation forms a crust on the host surface after drying.
Overgrowth: Excessive growth of the plant parts due to infection by pathogens. Overgrowth takes place by two processes
(i) Hyperplasia: abnormal increase in size due to excessively more cell division
(ii) Hypertrophy: abnormal increase in size or shape due to excessive enlargement of the size of cell of a particular tissue.
Atrophy: It is known as hypoplasia or dwarfing which is resulted from the inhibition of growth due to reduction in cell division or cell size.
Sclerotia: These are dark and hard structures of various shaped composed of dormant mycelia of some fungi. Sometimes, sclerotia are developed on the affected parts of the plant. Presence of sclerotia on the host surface is specifically called a sign of disease rather than symptom.
Development of epidemics:Sudden outbreak of a disease within a relatively short period covering a large areaand affecting many individuals in a population is called epidemic. Although, this term was originally designated to the human diseases, now applied in the diseases of animals, poultry, plants etc. Epidemic form of plant diseases is called as epiphytotics.
For a disease to occur, coincidence of three parameters of disease triangle is essential, namely, the vulnerable host, virulent pathogenand favourable environment. Under such circumstances, the pathogen not only completes its life cycle but also undergoes repeated generations. Then an epidemic develops only when few repeated generations are completed by the pathogen on the same host. As each generation or cycle of the pathogen takes a few days for completion, the fourth parameter i.e. time factor (forms a disease tetrahedron or disease pyramid) is also involved in epidemic build up.
In other words, epidemic growth is both temporal (pertaining to time) and a spatial(relating to space or area) process. The initial stages of an epidemic growth curve have a lag phase, when the incubation period is longer, inoculum load is weak and prevalent environmental conditions are unfavourable. Subsequently, when the conducive conditions occur, the growth of the disease is rapid and the severity of the epidemic explodes like a time bomb. Later, severity declines either due to unfavourable weather or crop maturity or both.
Plant Disease Management:
The word ‘control’ is a complete term where permanent ‘control’ of a disease is rarely achieved whereas, ‘management’ of a disease is a continuous process and is more practical in influencing adverse affect caused by a disease. Disease management requires a detail understanding of all aspects of crop production, economics, environmental, cultural, genetics and epidemiological information upon which the management decisions are made.
A. Principles of plant disease management:
There is six basic concept or principles or objectives lying under plant disease management.1. Avoidance of the pathogen: Occurrence of a disease can be avoided by planting/sowing a crop at times when, or in areas where, inoculum remain ineffective/inactive due to environmental conditions, or is rare or absent.
2. Exclusion of the pathogen: This can be achieved by preventing the inoculum from entering or establishing in a field or area when it does not exist. Legislative measures like quarantine regulations are needed to be strictly applied to prevent spread of a disease.
3. Eradication of the pathogen: It includes reducing, inactivating, eliminating or destroying inoculum at the source, either form a region or from an individual plant (rouging) in which it is already established.
4. Protection of the host: Host plants can be protected by creating a toxin barrier on the host surface by the application of chemicals.
5. Disease resistance: Preventing infection or reducing the effect of infection of the pathogen through the use of resistance host which is developed by genetic manipulation or by chemotherapy.
6. Therapy: Reducing severity of a disease in an infected individual. The first five principles are prophylactic (preventive) procedure and the last one is curative.
B. Methods of plant disease management
1. Avoidance of the pathogen:i. Choice of geographical area
ii. Selection of a field
iii. Adjustment of time of sowing
iv. Use of disease escaping varieties
v. Use of pathogen-free seed and planting material
vi. Modification of cultural practices
2. Exclusion of inoculum of the pathogen
i. Treatment of seed and plating materialsii. Inspection and certification
iii. Quarantine regulations
iv. Eradication of insect vector
3. Eradication of the pathogen
i. Biological control of plant pathogensii. Eradication of alternate and collateral hosts
iii. Cultural methods:
a. Crop rotation
b. Sanitation of field by destroying/burning crop debris
c. Removal and destruction of diseased plants or plant parts
d. Rouging iv. Heat and chemical treatment of diseased plants
v. Soil treatment: by use of chemicals, heat energy, flooding and fallowing
4. Protection of the host
i. Chemical control: application of chemicals (fungicides, antibiotics) by seed treatment, dusting and sprayingii. Chemical control of insect vectors
iii. Modifications of environment
iv. Modification of host nutrition
5. Disease resistance Use of resistant varieties:
Development of resistance in host is done byi. Selection and hybridization for disease resistance
ii. Chemotherapy
iii. Host nutrition
iv. Genetic engineering, tissue culture
6. Therapy Therapy of diseased plants can be done by
i. Chemotherapyii. Heat therapy
iii. Tree-surgery












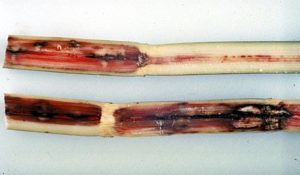 of the internode, especially when this red color is interrupted by occasional white patches extending crosswise of the stalk.
of the internode, especially when this red color is interrupted by occasional white patches extending crosswise of the stalk.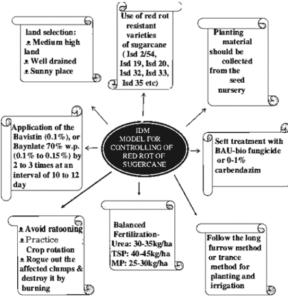
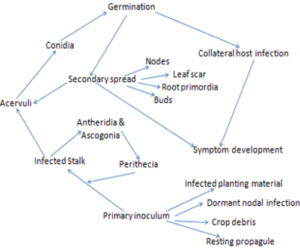


 lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion.
lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion. Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for
Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for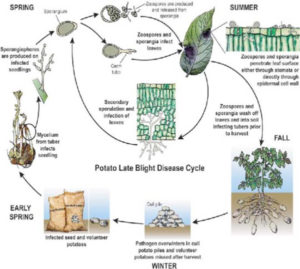
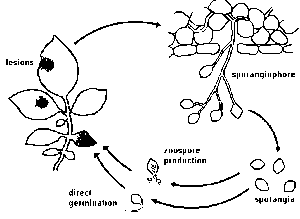
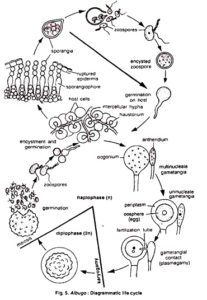
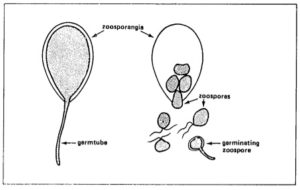 °C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.
°C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.