Late Blight of Potato| Phytophthora infestans| Symptoms & Control
Late blight is not only the most serious fungal disease of potatoes, it also occurs almost everywhere where potatoes are grown and is especially important in the traditional potato growing areas. On several occasions the disease has reached disastrous proportions. Well documented is the Irish Famine of around 1845 to 1850 when, as a result of a late blight epidemic, one million persons of a total of eight million inhabitants died and another 1.5 million left the country. The population of Ireland had completely depended on the potato that virtually formed its only food source. The biology of the disease and methods of its control were as yet entirely unknown. In other parts of Europe and in North America, the disease was as severe as in Ireland. In these areas, however, 3 famine was avoided due to a more varied food supply.
In spite of increased knowledge on the disease, late blight continues to be a major constraint to potato production worldwide. If not controlled, losses may reach 100 percent, and even lower infection levels may make the crop unfit for storage.
Under conditions of high humidity and cool temperatures, lesions expand rapidly. Sporulation may be visible at the lower surface of the leaves as a white mildew surrounding the lesions. it may become inconspicuous during the day, when the lesions dry and shrivel. In less than a week, the disease may spread from the first infected leaflets on a few plants to most plants in a field. Leaves may drop off.

 lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion.
lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion.
Tubers:
 Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for P. infestans into a badly smelling soft rot. Late blight normally does not spread during storage, but secondary infections may contaminate other tubers.
Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for P. infestans into a badly smelling soft rot. Late blight normally does not spread during storage, but secondary infections may contaminate other tubers.
Sporulation can be demonstrated in a simple experiment: A stem with small lesions on the leaves is placed in a flask with water. Stem and flask are covered with a plastic bag. It is kept overnight at a temperature between 15 and 22 °C (room temperature). Next morning the white sporulation, especially on the underside of the leaves, can be observed.
The size of zoosporangia is just below the detection limit of the unaided eye. Their lemon-shaped form can he observed with a microscope. Zoosporangia may germinate directly or indirectly.
To a small degree, zoosporangia germinate directly at temperatures above 20ºC (optimum 24 °C). Zoosporangia behave as single spores. They form germ tubes that penetrate into the plant tissue. In nature, direct germination seems of little importance.
Zoosporangia germinate indirectly at temperatures of 12 to 16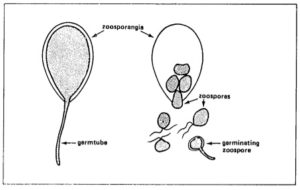 °C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.
°C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.
On leaves and stems, germ-tubes may directly penetrate the plant epidermis (no stomata are required). On tubers, germtubes penetrate through lenticels or wounds. Since the fungus cannot survive for a prolonged period outside the host tissue zoosporangia or zoospores die, if no suitable host tissue is available.
Formation of oospores helps P. infestans and related species to survive adverse conditions, such as the winter, dry periods, and absence of hosts. Possibly due to the absence of the mating type, oospore formation in P. infestanshas not been observed outside Mexico and Central America.
Oospores of P. infestans germinate through formation of sporangia, similar to those described in asexual reproduction. After infection of a host, the resulting zoospores may start a new life cycle.
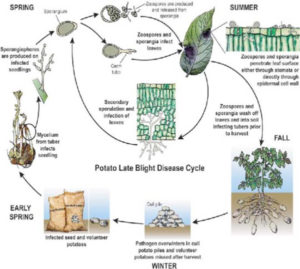
Infected seed tubers. In areas where potatoes are grown during defined seasons, diseased seed tubers are often the most important source of infection. Tubers become infected through lenticel and wounds when spores are washed into the soil by rain from infected leaves, especially when tubers are formed superficially or are not well covered by hilling. Also at harvest, tubers can become contaminated by contact with infected foliage.
Blight-infected tubers normally rot when planted in the field. However, few diseased tubers may form sprouts which then become primary sources of infection.
Cull piles. Infected tubers are frequently found in cull piles. Also tubers from previous crops that have been left in the field may be infected and may form ‘ source of primary infection for a new crop.
Neighboring potato fields. Neighboring potato fields are another source of infection especially in areas where potatoes are grown during the whole year.
Other host plants. Some other solanaceousplants may become affected by P. infestans. In many countries, the ornato is the most important alternative host. The pathogen also persists on wild species of potatoes that are widely distributed in the highlands of Mexico, Central and South America.
From primary infection sources, the wind disseminates the spores towards the fields. For development of the disease, temperature and humidity are of fundamental importance.
The most favorable temperature for development of the fungus (mycelium, sporangia) is around 21 °C, but the fungus remains alive in host tissues at air temperatures between 0 and at least 28 °C. Zoosporangia develop at temperatures between 9 and 22 °C.
Development of the fungus within the leaf is little affected by air humidity. However, sporangia are only formed when the relative humidity in the leaf canopy is above 95 percent. When the temperature is optimum, about eight hours of high hunlidity are required for production of zoosporangia, liberation of zoospores, and penetration. Water (dew, rain) must be on the leaf surface for at least two hours to allow zoospore formation, germination, and penetration.
In summary, the diease develops and spreadsmost rapidly at low temperatures and high humidity. Under these conditions, a disease cycle takes only three days. Unfavorable conditions may delay or temporarily interrupt the disease cycle.
For better disease management and the planning of a spray program, several methods of late blight forecasting have been developed, and are based on observation of temperature and humidity conditions. A severe late blight attack can be expected when the “leaf wetness period” exceeds 8 to 10 hours for several consecutive days, and temperatures range between 10 and 24 °C. Typical blight weather is characterized by cool, ,loudy days with frequent rains.
Disease forecasting programs appear to function generally better in temperate climates, than in most of the tropical highlands where potatoes are mainly grown in the rainy season.
Healthy seed. A basic condition for adequate crop production is the use of non-infected seed. This eliminates the primary infection source from the field.
Planting procedure. In regions with defined rainy seasons a change in planting time may help to reduce disease severity. This might however reduce the yield, as the potato requires abundant water during tuber formation.
Crop care. Any treatment that promotes rapid drying of foliage and reduces humidity within the crop helps to restrict disease development. These include wider planting distance and appropriate irrigation procedures. Sprinkler irrigation tends to increase disease severity.
Exposed tubers and those poorly covered by soil become readily infected by fungus spores washed down from the foliage.
Proper hilling reduces the amount of spores reaching the tubers and may result in faster drying of the field after a rain.
Resistance. Varieties with the highest resistance to late blight should be used, if commercially acceptable. Two types of resistance, race specific resistance and general resistance.
Harvest. When the foliage has been affected by late blight, it should be destroyed mechanically or Chemically at least one week before harvest. This practice reduces the chance of tuber infection by contact with infected leaves and stems and promotes skin suberization, making tubers less vulnerable to infection. In addition, this helps to reduce mechanical damage and infection by storage pathogens.
Tubers should be only harvested when they are mature (the skin should not be peeling). The soil should be dry to present infection through damaged skin or lenticels. Only disease-free tubers should be stored.
Crop residues, including infected tubers, should be removed from the field or plowed under. Cull piles should be covered with sufficient soil to prevent emergence of discarded tubers.
Fungicide applications. Preventive fungicides principally inhibit spore germination and penetration. Once the pathogen enters the leaves, most fungicides are ineffective. A preventive fungicide application program should start immediately after the first blight symptoms appear in a crop. Proper fungicide cover on the foliage should be maintained as long as favorable late blight conditions exist. In a susceptible crop, up to 15 applications per season may be required.
Proper dosage and application are as important selecting an effective fungicide.
Instructions given with the fungicide should be followed carefully. The spraying equipment should be kept in good condition to Lssure an even and fine spray application.
Observation of meteorological data may help determine when fungicide applications are necessary.
Newly developed systemic fungicides (acylalanines) are translocated within the plant. A constant and uniform coverage is less critical than with conventional fungicides, and frequency of spraying may be reduced. Granular formulations applied to the soil do not require spraying equipment. Systemic fungicides may be highly effective; however, development of resistance of the fungus to these fungicides has been reported recently.
No general recommendations for fungicide application can be given, since availability, effectivity, conditions, and regulations vary from country to country. Local experts should be consulted for recommendations in a particular area.
Integrated control. The best control is a combination of preventive measures, based on the use of resistant varieties. Costly fungicide applications can then be reduced. The objective of integrated management of late blight is not the eradication of the disease, but the most economical production of a potato crop.
In spite of increased knowledge on the disease, late blight continues to be a major constraint to potato production worldwide. If not controlled, losses may reach 100 percent, and even lower infection levels may make the crop unfit for storage.
SYMPTOMS:
In an advanced stage of disease development, symptoms resemble those caused by frost attack. Plants severely affected with late blight produce a distinctive odor, which results from the breakdown of plant tissue. The disease affects leaves, stems, and tubers.Leaves.
The earliest symptoms of the disease are often present on lower leaves. They consist of small, pale to dark green spots the, change into brown or black lesions, depending on the humidity of the air. Lesions begin frequently at leaf tips and margins. A pale green or yellow border, a few millimeters wide, often separates dead from healthy tissue.Under conditions of high humidity and cool temperatures, lesions expand rapidly. Sporulation may be visible at the lower surface of the leaves as a white mildew surrounding the lesions. it may become inconspicuous during the day, when the lesions dry and shrivel. In less than a week, the disease may spread from the first infected leaflets on a few plants to most plants in a field. Leaves may drop off.

Stems:
Either by direct infection or extending from the leaves, lesions may develop on petioles and stems, where they expand lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion.
lengthwise. Infected stems are weakened, and may collapse thereby causing death of plant parts above the lesion.Tubers:
 Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for P. infestans into a badly smelling soft rot. Late blight normally does not spread during storage, but secondary infections may contaminate other tubers.
Infected tubers show a superficial and irregular discoloration. Dry and brown necrotic lesions penetrate from the surface into the tuber tissue. Secondary pathogens (mainly bacteria) may convert the almost odorless(faint vinegar smell) dry rot typical for P. infestans into a badly smelling soft rot. Late blight normally does not spread during storage, but secondary infections may contaminate other tubers.Life Cycle of P. infestans
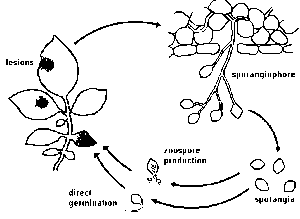
Most stages of the life cycle of P. infestans can only be seen with the aid of a microscope. In this way, fungal threads (mycelium) can easily be observed. They are characterized by the absence of cross walls (septa). The mycelium develops between cells (intercellularly) and only extensions of it (haustoria) enter the cells. Both asexual (vegetative) and sexual (generative) reproduction occurs.Asexual reproduction.
Between three and ten days after infection, depending on environmental conditions, spore-bearing organs (sporangiophores)emerge through openings (stomata) on the leaf surface. Stomata are more frequent on the underside than on the upperside of leaves. This explains why sporulation on the underside is more abundant than on the upperside. Zoosporangia (also called sporangia or conidia) develop at the end of these sporangiophores. When mature, the zoosporangia easily break off and are spread by the wind. Most spores are deposited after a few meters, however, travel distances of over 30 km have been reported.

Sporulation can be demonstrated in a simple experiment: A stem with small lesions on the leaves is placed in a flask with water. Stem and flask are covered with a plastic bag. It is kept overnight at a temperature between 15 and 22 °C (room temperature). Next morning the white sporulation, especially on the underside of the leaves, can be observed.
The size of zoosporangia is just below the detection limit of the unaided eye. Their lemon-shaped form can he observed with a microscope. Zoosporangia may germinate directly or indirectly.
To a small degree, zoosporangia germinate directly at temperatures above 20ºC (optimum 24 °C). Zoosporangia behave as single spores. They form germ tubes that penetrate into the plant tissue. In nature, direct germination seems of little importance.
Zoosporangia germinate indirectly at temperatures of 12 to 16
 °C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.
°C,each sporangium releasing from 10 to 20 swarm spores (zoospores). Activated by two flagellae, zoospores remain motile from a few minutes up to several hours. Under certain conditions they lose the flagellae, form a cell wall and subsequently a germ tube.On leaves and stems, germ-tubes may directly penetrate the plant epidermis (no stomata are required). On tubers, germtubes penetrate through lenticels or wounds. Since the fungus cannot survive for a prolonged period outside the host tissue zoosporangia or zoospores die, if no suitable host tissue is available.
Sexual reproduction:
Until 1984, the sexual stage of P. infestans has only been reported in Mexico and parts of Central America. More recent reports inform about its occurrence in other parts of the world. When mycelia of different types of the fungus (called mating types A1 and A2) grow together, one of them may form male cells (antheridia) and the other female cell! (oogonia). The oogonium grows through the antheridium, allowing fertilization. The fertilized oogonium develops into a thick-walled resting spore (oospore). Oospores, in contrast to zoosporangia and zoospores, can resist unfavorable conditions, such as droughts and low temperatures.Formation of oospores helps P. infestans and related species to survive adverse conditions, such as the winter, dry periods, and absence of hosts. Possibly due to the absence of the mating type, oospore formation in P. infestanshas not been observed outside Mexico and Central America.
Oospores of P. infestans germinate through formation of sporangia, similar to those described in asexual reproduction. After infection of a host, the resulting zoospores may start a new life cycle.
EPIDEMIOLOGY :
With the exception of oospores which may survive in the soil, in nature the pathogen persists only in susceptible hosts. Sources of infection are:” infected seed tubers, ” cull piles, ” neighboring potato fields, ” other host plants.Infected seed tubers. In areas where potatoes are grown during defined seasons, diseased seed tubers are often the most important source of infection. Tubers become infected through lenticel and wounds when spores are washed into the soil by rain from infected leaves, especially when tubers are formed superficially or are not well covered by hilling. Also at harvest, tubers can become contaminated by contact with infected foliage.
Blight-infected tubers normally rot when planted in the field. However, few diseased tubers may form sprouts which then become primary sources of infection.
Cull piles. Infected tubers are frequently found in cull piles. Also tubers from previous crops that have been left in the field may be infected and may form ‘ source of primary infection for a new crop.
Neighboring potato fields. Neighboring potato fields are another source of infection especially in areas where potatoes are grown during the whole year.
Other host plants. Some other solanaceousplants may become affected by P. infestans. In many countries, the ornato is the most important alternative host. The pathogen also persists on wild species of potatoes that are widely distributed in the highlands of Mexico, Central and South America.
From primary infection sources, the wind disseminates the spores towards the fields. For development of the disease, temperature and humidity are of fundamental importance.
The most favorable temperature for development of the fungus (mycelium, sporangia) is around 21 °C, but the fungus remains alive in host tissues at air temperatures between 0 and at least 28 °C. Zoosporangia develop at temperatures between 9 and 22 °C.
Development of the fungus within the leaf is little affected by air humidity. However, sporangia are only formed when the relative humidity in the leaf canopy is above 95 percent. When the temperature is optimum, about eight hours of high hunlidity are required for production of zoosporangia, liberation of zoospores, and penetration. Water (dew, rain) must be on the leaf surface for at least two hours to allow zoospore formation, germination, and penetration.
In summary, the diease develops and spreadsmost rapidly at low temperatures and high humidity. Under these conditions, a disease cycle takes only three days. Unfavorable conditions may delay or temporarily interrupt the disease cycle.
For better disease management and the planning of a spray program, several methods of late blight forecasting have been developed, and are based on observation of temperature and humidity conditions. A severe late blight attack can be expected when the “leaf wetness period” exceeds 8 to 10 hours for several consecutive days, and temperatures range between 10 and 24 °C. Typical blight weather is characterized by cool, ,loudy days with frequent rains.
Disease forecasting programs appear to function generally better in temperate climates, than in most of the tropical highlands where potatoes are mainly grown in the rainy season.
CONTROL :
Development of late blight is favored when the potato variety is susceptible, and appropriate environmental conditions persist for sufficient time. Generally, any agronomic measure that affects this relationship may be utilized to reduce the disease.Healthy seed. A basic condition for adequate crop production is the use of non-infected seed. This eliminates the primary infection source from the field.
Planting procedure. In regions with defined rainy seasons a change in planting time may help to reduce disease severity. This might however reduce the yield, as the potato requires abundant water during tuber formation.
Crop care. Any treatment that promotes rapid drying of foliage and reduces humidity within the crop helps to restrict disease development. These include wider planting distance and appropriate irrigation procedures. Sprinkler irrigation tends to increase disease severity.
Exposed tubers and those poorly covered by soil become readily infected by fungus spores washed down from the foliage.
Proper hilling reduces the amount of spores reaching the tubers and may result in faster drying of the field after a rain.
Resistance. Varieties with the highest resistance to late blight should be used, if commercially acceptable. Two types of resistance, race specific resistance and general resistance.
Harvest. When the foliage has been affected by late blight, it should be destroyed mechanically or Chemically at least one week before harvest. This practice reduces the chance of tuber infection by contact with infected leaves and stems and promotes skin suberization, making tubers less vulnerable to infection. In addition, this helps to reduce mechanical damage and infection by storage pathogens.
Tubers should be only harvested when they are mature (the skin should not be peeling). The soil should be dry to present infection through damaged skin or lenticels. Only disease-free tubers should be stored.
Crop residues, including infected tubers, should be removed from the field or plowed under. Cull piles should be covered with sufficient soil to prevent emergence of discarded tubers.
Fungicide applications. Preventive fungicides principally inhibit spore germination and penetration. Once the pathogen enters the leaves, most fungicides are ineffective. A preventive fungicide application program should start immediately after the first blight symptoms appear in a crop. Proper fungicide cover on the foliage should be maintained as long as favorable late blight conditions exist. In a susceptible crop, up to 15 applications per season may be required.
Proper dosage and application are as important selecting an effective fungicide.
Instructions given with the fungicide should be followed carefully. The spraying equipment should be kept in good condition to Lssure an even and fine spray application.
Observation of meteorological data may help determine when fungicide applications are necessary.
Newly developed systemic fungicides (acylalanines) are translocated within the plant. A constant and uniform coverage is less critical than with conventional fungicides, and frequency of spraying may be reduced. Granular formulations applied to the soil do not require spraying equipment. Systemic fungicides may be highly effective; however, development of resistance of the fungus to these fungicides has been reported recently.
No general recommendations for fungicide application can be given, since availability, effectivity, conditions, and regulations vary from country to country. Local experts should be consulted for recommendations in a particular area.
Integrated control. The best control is a combination of preventive measures, based on the use of resistant varieties. Costly fungicide applications can then be reduced. The objective of integrated management of late blight is not the eradication of the disease, but the most economical production of a potato crop.











0 comments:
Post a Comment